Electrolysis
Electrolysis Revision
Electrolysis
Electrolysis is a process used to separate chemicals using electricity. This process is performed on solutions containing charged particles such as ions. Electrolysis can be used to achieve a number goals, such as extracting metals from their ores and separating ionic compounds.
The Process of Electrolysis
Electrolysis is a chemical process that can be used to separate ionic compounds into their constituent ions, when in solution or when molten, by using an electrical current to drive chemical reactions. Electrolysis makes use of electrostatic attractions to pull the ions in a solution in opposite directions. It is from this that we get the word electrolysis, which means to split using electricity. Substances must be in solution or molten to undergo electrolysis as the ions of the substance need to be able to move. If the substances were solid then the ions within them would be bound in place and unable to separate.
To electrolyse an ionic compound a positive electrode, known as the anode, and a negative electrode, known as the cathode, should be immersed in a solution of the compound, known as the electrolyte. The electrodes are then connected to an electrical power source, allowing a circuit to be set up. In most cases these electrodes are inert (meaning that they do not react). This prevents the electrolyte from reacting with the electrodes. A current will flow from the power source to the electrodes, with the electrolyte carrying charge between them to complete the circuit. This causes the ions of the electrolyte to move towards them, causing the compound to decompose.
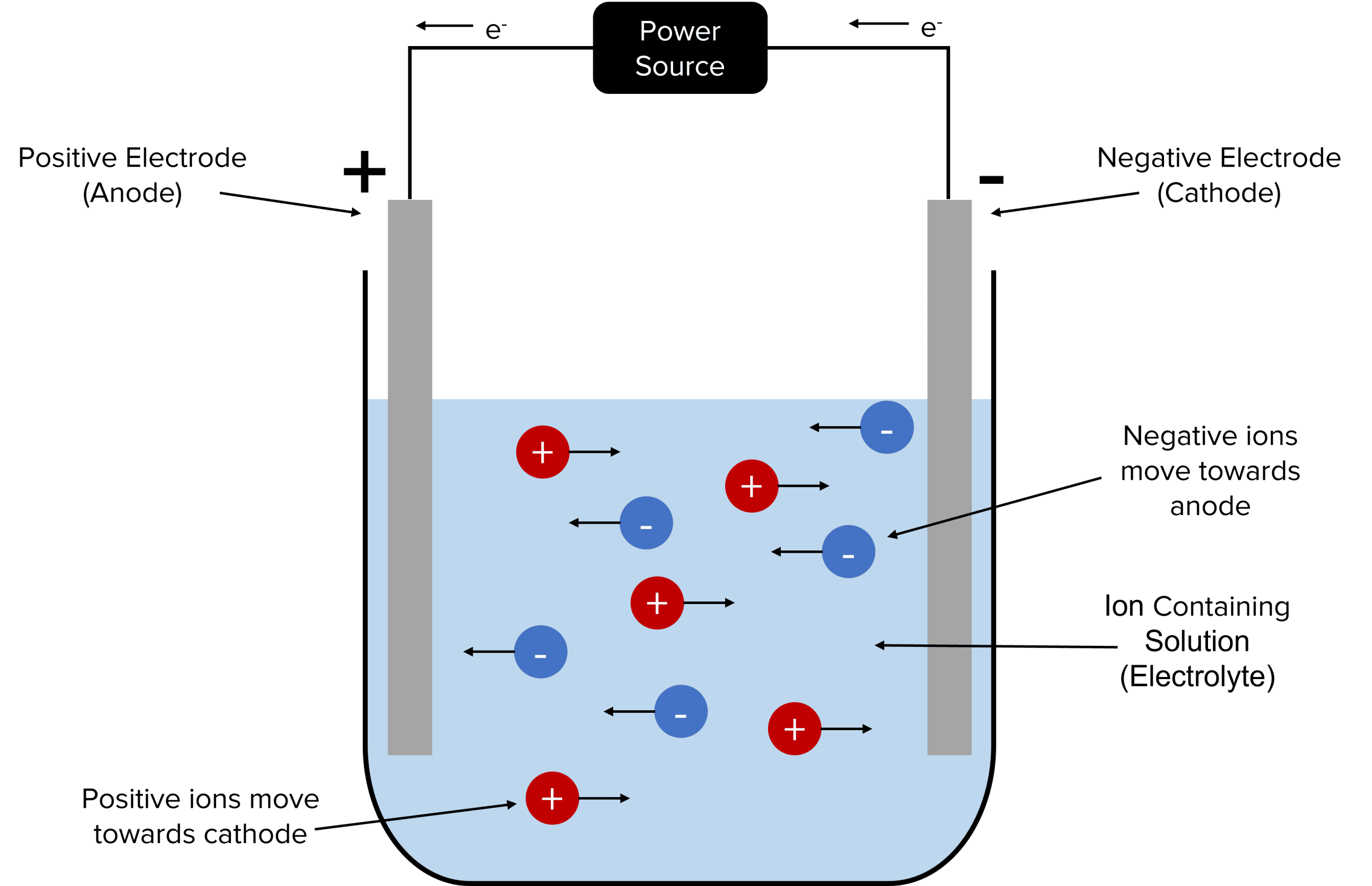
The positive ions of the electrolyte will be attracted to the negative charge of the cathode. This causes the ions to move through the solution towards the cathode. The positive ions are then reduced at the cathode by electrons flowing in from the external circuit.
The negative ions of the electrolyte will be attracted to the positive charge of the anode. This causes the ions to move through the solution towards the anode. The negative ions are then oxidised at the anode. The electrons that are released by this process will then be collected by the cathode to flow into the external circuit.
Once an ion has been oxidised or reduced it is discharged from the solution. Discharged species will often leave the solution either as solid sediments or as gasses.
Metal Extraction
One useful application of electrolysis is the extraction of metals from their ores when these metals are too reactive to be reduced by carbon. Take for example the extraction of aluminium from its ore bauxite. Bauxite has the chemical formula \text{Al}_2\text{O}_3 and can be electrolysed when molten. The melting point of bauxite is very high, so it is mixed with another compound called cryolite which reduces bauxites melting point.
Once molten, the bauxite is then electrolysed using a pair of graphite electrodes.
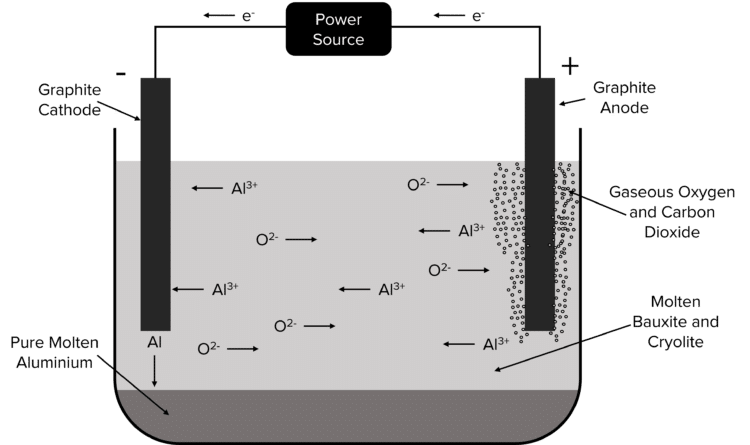
The positive \text{Al}^{3+} ions are attracted to the negative graphite cathode. Here they are reduced by electrons flowing in from the external power source. This produces molten elemental \text{Al}. This molten aluminium is heavier than the mixture of bauxite and cryolite and falls to the bottom of the mixture where it can be collected.
The negative \text{O}^{2-} ions are attracted to the positive graphite anode. Here they are oxidised to form gaseous \text{O}_2, giving up their electrons to the external circuit. The \text{O}^{2-} ions may also react with the carbon of the graphite anode to produce gaseous \text{CO}_2.
Electrolysis of Aqueous Solutions
When aqueous solutions are used as electrolytes the picture becomes more complicated than that of the electrolysis of molten ionic compounds. When molten compounds are electrolysed the only ions produced are those of the molten compound. When aqueous solutions are electrolysed, the water in the solution will also electrolyse to form \text{H}^{+} and \text{OH}^{-} ions in the following reversible reaction.
\text{H}_2\text{O}_{\text{(l)}}\rightleftharpoons\text{H}^{+}_{\text{ (aq)}}+\text{OH}^{-}_{\text{ (aq)}}
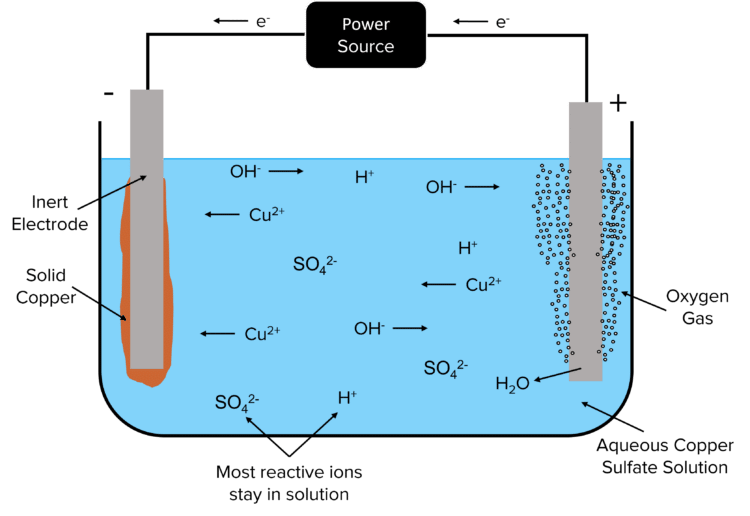
The reactions that take place at the electrodes will then depend on the reactivity of the ionic species involved. Take for example the electrolysis of copper sulfate. As copper metal is less reactive than hydrogen, \text{Cu}^{2+} ions will react at the cathode in preference to hydrogen and copper metal is produced. As there are no halide ions present in the solution, \text{OH}^{-} ions will react at the anode in preference to the sulfate ions. This produces water and oxygen gas.
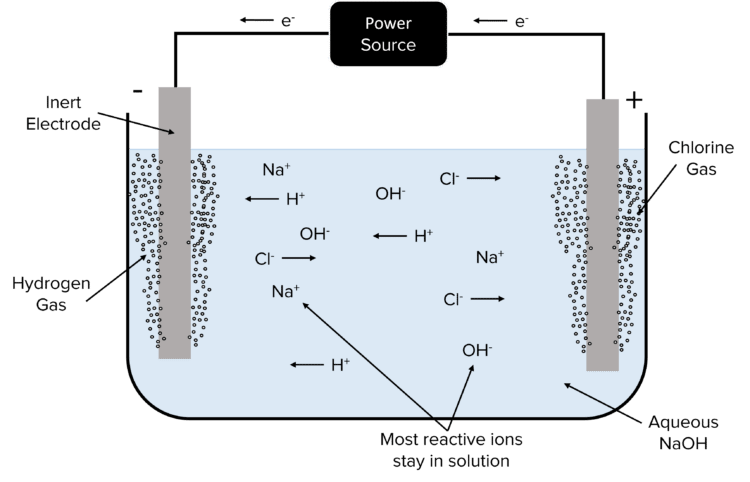
Another example is that of the electrolysis of \text{NaCl}. In this case, sodium is more reactive than hydrogen and so hydrogen reacts at the cathode in preference. This produces hydrogen gas. The solution also contains halide ions in the form of \text{Cl}^{-} ions. These will react at the anode in preference to the hydroxide ions, producing chlorine gas.
Electrode Half Equations
As has been seen above, one of the features of electrolysis is the presence of chemical reactions at each electrode. These reactions take the form of redox half reactions, that contribute to an overall electrolytic reaction. Take for example the extraction of aluminium from its oxides given above. In this process there is an overall decomposition reaction, where by bauxite breaks down into pure aluminium and oxygen:
2\text{Al}_2\text{O}_{3\text{(l)}}\rarr4\text{Al}_{\text{(l)}}+3\text{O}_{2\text{(g)}}
However, the break down of bauxite by electrolysis does not directly produce either of these products. Instead, ions of aluminium and oxygen are produced and then reduced or oxidised at the electrodes.
The aluminium ions \left(\text{Al}^{3+}\right) are reduced at the cathode to form pure aluminium. It is possible to write a half equation for this reduction to show the formation of elemental aluminium (the term \text{e}^{-} is used to represent the electrons).
\text{Al}^{3+}_{ \text{(l)}}+3\text{e}^{-}\rarr\text{Al}_{\text{(l)}}
The \text{O}^{2-} ions are oxidised at the anode to form oxygen gas. It is again possible to write a half equation for this process.
2\text{O}^{2-}_{\text{ (g)}}\rarr\text{O}_{2\text{(g)}}+4\text{e}^{-}
We can double check that a half equation is correct by checking if charge is balanced across the equation. The charges should be equal on both sides of the reaction (e.g. above there is a total charge of -4 on the left and a total charge of -4 on the right).
Required Practical
Testing for the products of electrolysis
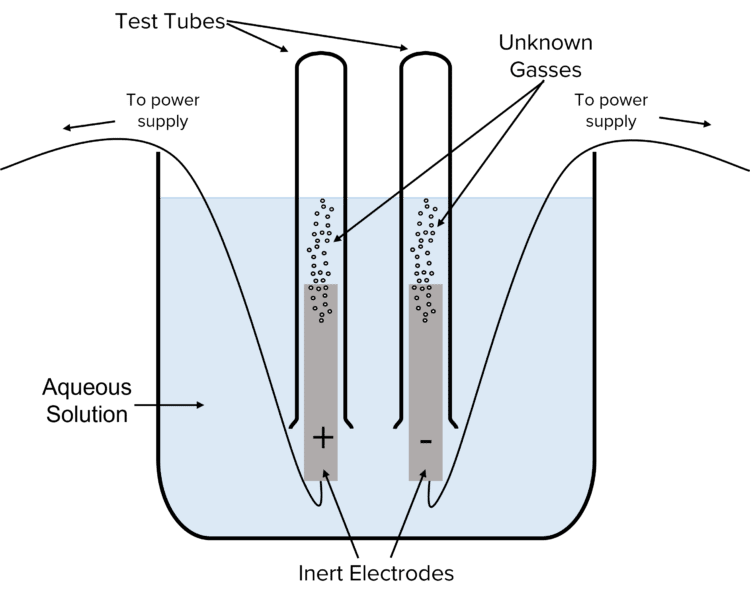
It is possible to carry out tests for the ions present in electrolytes by collecting the gasses produced by electrolysis reactions in inverted test tubes.
Method
- Chose an electrolyte that may or may not contain halide ions.
- Pour out a sample of the electrolyte into a large beaker.
- Immerse two inert electrodes into the solution and place an inverted test tube over each electrode.
- Connect the electrodes to a power supply to electrolyse the electrolyte.
- Continue to apply the power until no more gas is produced in the test tubes.
Chemical Tests
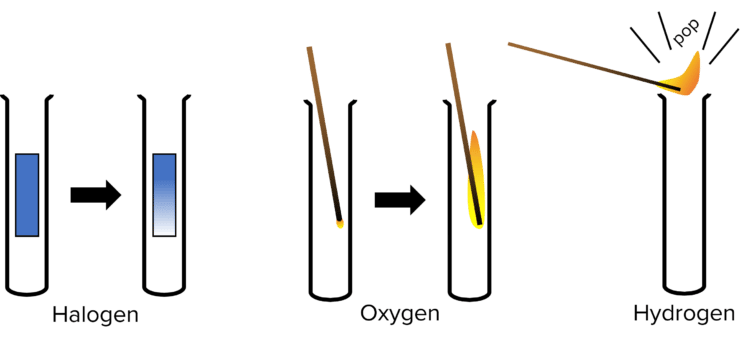
Now the gaseous products of the electrolysis have been collected in the test tubes, we can perform chemical tests on them to determine which gasses they are.
- To test for halogen gasses we can use damp, blue litmus paper. If there is any chlorine gas present in the test tube then holding the litmus paper inside the test tube should cause it to be bleached and will turn white.
- To test for oxygen we can use a splint. Light a splint and then blow it out. Quickly put the glowing splint into the test tube. If the splint reignites then oxygen is present.
- To test for hydrogen we can use a lit split. Hold the flame of a lit splint over the test tube. If a squeaky popping sound is produced than hydrogen gas is present.
Electrolysis Example Questions
Question 1: A solution of aqueous copper chloride is electrolysed. This produces chlorine gas and solid copper. State the electrode at which you would expect to find solid copper.
[1 mark]
The cathode.
Question 2: Explain why the electrodes used in electrolysis are typically inert.
[1 mark]
So that they do not react with the electrolyte.
Question 3: Describe a test for hydrogen gas.
[4 marks]
- Light a splint.
- Hold the flame of the splint over a test tube suspected of containing hydrogen gas.
- If a squeaky pop is produced, hydrogen gas is present.
Question 4: Give the products of the electrolysis of molten bauxite \left(\text{Al}_2\text{O}_3\right).
[3 marks]
\text{Al}_{\text{(l)}}
\text{O}_{2\text{(g)}}
\text{CO}_{2\text{(g)}}
(State symbols not necessary for marks)
Question 5: Give the half equations for the production of solid copper and chlorine gas in the electrolysis of copper chloride.
[4 marks]
\text{Cu}^{2+}_{\text{(aq)}}+2\text{e}^{-}\rarr\text{Cu}_{\text{(s)}}
2\text{Cl}^{-}_{\text{(aq)}}\rarr\text{Cl}_{2\text{(g)}}+2\text{e}^{-}
(State symbols not necessary for marks. Two marks per correct half equation.)






